MyoKardia Announces 48-week Data from PIONEER-OLE Study of Mavacamten

SOUTH SAN FRANCISCO, Calif., Nov. 11, 2019 (GLOBE NEWSWIRE) -- MyoKardia, Inc. (Nasdaq: MYOK) today announced new data from the company’s PIONEER open-label extension (OLE) study of mavacamten for the treatment of symptomatic, obstructive hypertrophic cardiomyopathy (HCM).
Data for twelve patients at 48 weeks of treatment with mavacamten were consistent with prior safety and efficacy observations at the 12, 24, and 36-week readouts. Highlights of the data include continued safety and tolerability and sustained clinical benefits, including reductions in left ventricular outflow tract (LVOT) gradient, improvements in NYHA functional class and improvement of multiple biomarkers toward normal ranges. A reduction in septal wall thickness, a defining characteristic of HCM, as well as an improvement in patient reported quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), were also reported.
“Mavacamten’s continued favorable safety profile and the apparent consistency and durability across so many parameters through one year of treatment are incredibly encouraging and enhance our confidence that mavacamten has the potential to make a lasting impact on the lives of people with HCM. We look forward to building on these data with the results from our pivotal EXPLORER-HCM Phase 3 study in the first half of next year,” said Jay Edelberg, M.D., Ph.D., Senior Vice President of Clinical Development at MyoKardia.
“The hearts of people with HCM beat as if they are in fight-or-flight mode all the time, and as disease progresses, that excessive contraction can result in a cascade of damaging, and ultimately fatal, consequences for HCM patients. Mavacamten acts at a molecular level on specific heart muscle proteins to reduce the excessive contractility driving disease,” said Daniel Jacoby, M.D. Director, Comprehensive Heart Failure Program at the Yale School of Medicine and an investigator in the PIONEER-OLE clinical trial. “Over time, and across multiple measures, we are seeing evidence that mavacamten treatment is bringing the HCM heart toward a more normal state. The new data being reported during the AHA sessions, including the improvement of patient-reported outcomes and heart structure, add further weight to the sustained safety and efficacy observed to date in this study population.”
PIONEER-OLE 48-week Results
Twelve patients with symptomatic (NYHA Class II-III), obstructive HCM are currently enrolled in the PIONEER-OLE study and receive individualized doses of mavacamten aimed at reducing or eliminating their LVOT obstruction. All twelve patients were evaluable at 48 weeks.
- Mavacamten was well tolerated throughout the one-year treatment period. Consistent with data reported at Week 36, there were no cardiac-related adverse events (AEs) attributed to study drug throughout the 48-week period. To date, all AEs attributed to treatment have been mild or moderate and transient.
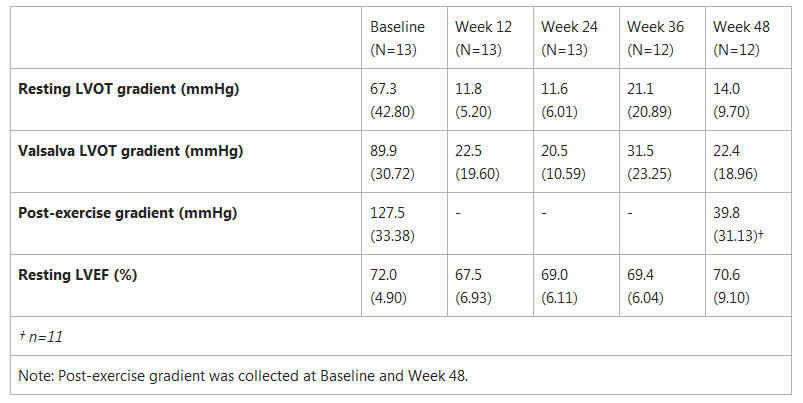
- LVOT gradient, a measure of obstruction of the left ventricle, was decreased from baseline with statistical significance among the twelve patients under multiple conditions of testing: i.e. at rest, post-exercise and upon provocation with a Valsalva maneuver. At week 48, resting LVOT gradient for all patients was below 50mmHg, the guideline-based threshold for an invasive intervention, and 11 of 12 patients were below the 30mmHg threshold at which obstructive HCM is diagnosed. Provoked gradient measurements, taken using a Valsalva maneuver and post-exercise, were also below 50mmHg in all but two patients at Week 48.
- Left ventricular ejection fraction (LVEF) remained above normal (50%) for all 12 patients at all times of assessment.
PIONEER-OLE: Observed Mean Values (SD)
LVOT Gradient and LVEF
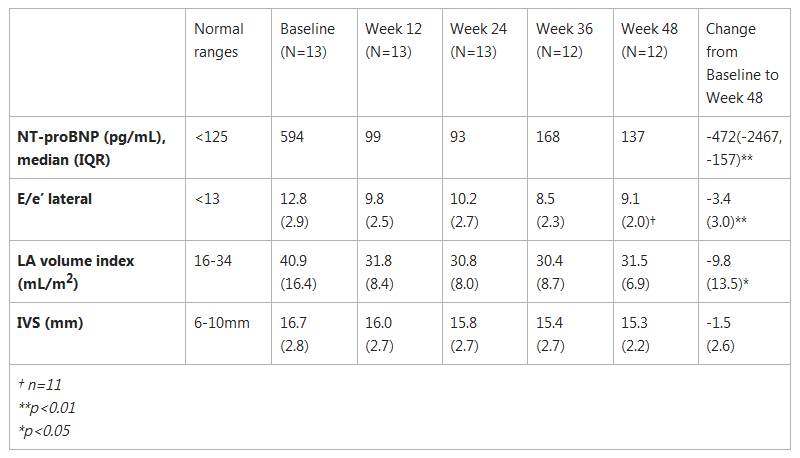
- NT-proBNP, an established circulating blood marker of cardiac wall stress, significantly decreased to ranges closer to normal (considered less than 125 pg/mL). NT-proBNP levels in HCM patients of <310 pg/mL have been associated with a 75 percent reduction in the rate of heart failure-related death or hospitalization, progression to end-stage disease, and stroke, as compared with patients with levels ≥310 pg/mL.(1)
- E/e’, an echocardiographic measure of left ventricular filling pressure, decreased from a mean baseline measure of 12.8 to 9.1.
- Left atrial volume index decreased to normal levels from a baseline mean of 41 mL/m2 to a mean of 32 mL/m2. Left atrial volumes are a measure of the filling pressure of the left ventricle, and increased volumes are potentially associated with an increased risk of atrial fibrillation in HCM patients.(2)
- Reductions in interventricular septal (IVS) thickness as measured by echocardiography were observed in PIONEER-OLE patients. Overall, PIONEER-OLE patients began the study with a mean IVS of 17mm at baseline, and progressively decreased to 15mm after 48 weeks of mavacamten treatment. Studies of HCM patients following septal reduction interventions have shown that IVS reductions in HCM patients are associated with improvements in LVOT gradient, functional capacity and symptoms. The risk of sudden cardiac death in HCM patients has been observed to increase progressively as wall thickness increases above 15mm.(3)
PIONEER-OLE: Biomarker Measurements, Mean (SD)
Cardiac Wall Stress, Diastolic Filling Pressure and Structural Changes
Improvements in both symptom burden and quality of life has been observed among the PIONEER-OLE patients.
- At baseline, patients enrolled in PIONEER-OLE were symptomatic with a NYHA classification of Class II or III. NYHA classifications were measured at Week 24 and Week 48 and have consistently demonstrated improvements, with nine out of twelve patients achieving asymptomatic (Class I) status.
- Positive results from the KCCQ, designed to measure patients’ perception of their heart failure health status and its impact on the activities of daily living, were also reported. In PIONEER-OLE, KCCQ mean scores went from 74.1 at baseline to 87.3 at Week 48 (scores range from 0-100, and higher scores reflect better status). A clinically significant change in KCCQ is defined as greater than or equal to 6.
These data will be presented at the 2019 American Heart Association’s Annual Scientific Sessions by Stephen Heitner, M.D., Director of the Hypertrophic Cardiomyopathy Clinic at Oregon Health and Science University, on Monday, November 18, 2019 at 2:05 p.m. during the session titled: Pharmacological Therapy in HF/Cardiomyopathy: The Next Important Indication or Agent? (Article from : www.drugs.com)

