FDA Approves Kebilidi (eladocagene exuparvovec-tneq) Gene Therapy for the Treatment of AADC Deficiency
WARREN, N.J., Nov. 13, 2024 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) announced today the U.S. Food and Drug Administration (FDA) accelerated approval of its gene therapy for the treatment of AADC deficiency, the first-ever gene therapy approved in the United States that is directly administered to the brain.
"PTC has once again pioneered a new approach to treating highly morbid neurologic diseases," said Matthew B. Klein, M.D., Chief Executive Officer, PTC Therapeutics. "I am proud of our team's unwavering commitment to achieve this important regulatory milestone. We look forward to bringing this transformational gene therapy to children and adults with AADC deficiency in the United States."
The gene therapy, which will be marketed in the United States with the brand name Kebilidi™ (eladocagene exuparvovec-tneq), is indicated for the treatment of children and adults with AADC deficiency, including the full spectrum of disease severity. Launch preparations are well underway, with centers of excellence already identified and surgeons trained in the procedure to deliver the gene therapy.
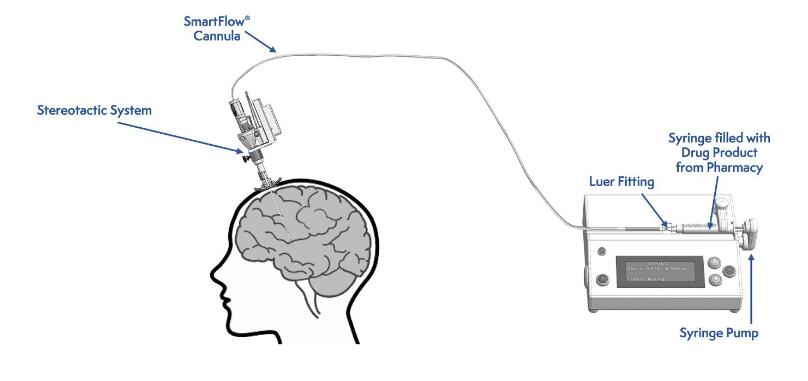
AADC deficiency is a highly morbid and life-shortening rare genetic disorder that results in the inability to synthesize dopamine, a neurotransmitter essential for motor function. Kebilidi is a gene replacement therapy that is directly administered to the putamen of the brain through a stereotactic neurosurgical procedure. Clinical trial results demonstrate that following gene therapy, de novo synthesis of dopamine occurs, followed by the progressive acquisition of motor development milestones.
Kebilidi received accelerated approval based on the safety and clinical efficacy findings in the ongoing global clinical trial of the gene therapy (PTC-AADC-GT-002). Confirmatory evidence will be provided from the long-term follow up of patients already treated in the study.
Along with the Biologics License Application approval, a Rare Disease Priority Review Voucher was granted. The company plans to monetize the voucher.
About aromatic L-amino acid decarboxylase (AADC) deficiency
AADC deficiency is a fatal, rare genetic disorder that typically causes severe disability and suffering from the first months of life, affecting every aspect of life—physical, mental and behavioral.1-4 The suffering of children with AADC deficiency may be exacerbated by oculogyric crises, which are distressing, painful episodes that resemble seizures and are characterized by a stuck upward gaze, dystonia, and inconsolability.1-5
The lives of affected children are severely impacted and shortened.1 Ongoing physical, occupational and speech therapy, and interventions, including surgery, are also often required to manage potentially life-threatening complications, such as infections and severe feeding and breathing problems.1,6,7
About Kebilidi™ (eladocagene exuparvovec-tneq)
Kebilidi is a recombinant adeno-associated virus serotype 2 (rAAV2)-based gene therapy, containing the human DDC gene. It is designed to correct the underlying genetic defect by delivering a functioning DDC gene directly into the putamen, increasing the AADC enzyme and restoring dopamine production.
Administration of Kebilidi occurs through a stereotactic surgical procedure, a minimally invasive neurosurgical procedure used for the treatment of a number of pediatric and adult neurological disorders. The administration procedure is performed by a qualified neurosurgeon in centers specialized in stereotactic neurosurgery. Kebilidi is authorized to be administered using the SmartFlow Neuro Cannula.
Indications and Important Safety Information
Indication
Kebilidi is an adeno-associated virus vector-based gene therapy indicated for the treatment of adult and pediatric patients with aromatic L-amino acid decarboxylase (AADC) deficiency. This indication is approved under accelerated approval based on change from baseline in gross motor milestone achievement at 48 weeks post-treatment. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial.
IMPORTANT SAFETY INFORMATION
Contraindications
Kebilidi is contraindicated in patients who have not achieved skull maturity assessed by neuroimaging. Skull maturity is needed for stereotactic neurosurgical administration of Kebilidi.
Warnings and Precautions
Procedure-Related Monitoring: Procedural complications have been reported after neurosurgery required for Kebilidi administration. These events included respiratory and cardiac arrest which occurred within 24 hours of the neurosurgical procedure and during post-surgical care. Kebilidi administration has the potential risk for additional procedure related adverse events including cerebrospinal fluid (CSF) leak, intracranial bleeding, neuroinflammation, acute infarction, and infection. Patients should be monitored for procedure related adverse events with Kebilidi administration.
Dyskinesia: Dyskinesia was reported after administration of Kebilidi. All events were reported within 3 months of administration and 2 events required hospitalization. Monitor patients for signs and symptoms of dyskinesia after Kebilidi treatment which may include involuntary movements of face, arm, leg, or entire body. These may present as fidgeting, writhing, wriggling, head bobbing or body swaying. The use of dopamine antagonists may be considered to control dyskinesia symptoms.
Adverse Reactions
The most common adverse reactions (≥15%) in patients treated with Kebilidi were dyskinesia (77%), pyrexia (38%), hypotension (31%), anemia (31%), salivary hypersecretion (23%), hypokalemia (23%), hypophosphatemia (23%), insomnia (23%), hypomagnesemia (15%), and procedural complications, including respiratory and cardiac arrest (15%).
Use in Specific Populations
- Pediatric Use: The safety and efficacy of Kebilidi have not been studied in pediatric patients younger than 16 months.
- Geriatric Use: Clinical studies of Kebilidi did not include patients 65 years of age and older.
You may also report adverse events directly to FDA at 1–800–FDA–1088 or www.fda.gov/medwatch. For product complaints or to report an adverse event, please call 1–866–562–4620 or email at usmedinfo@ptcbio.com.
For more additional information, please see the full Prescribing Information.
About PTC Therapeutics, Inc.
PTC is a global biopharmaceutical company focused on the discovery, development and commercialization of clinically differentiated medicines that provide benefits to children and adults living with rare disorders. PTC's ability to innovate to identify new therapies and to globally commercialize products is the foundation that drives investment in a robust and diversified pipeline of transformative medicines. PTC's mission is to provide access to best-in-class treatments for patients who have little to no treatment options. PTC's strategy is to leverage its strong scientific and clinical expertise and global commercial infrastructure to bring therapies to patients. PTC believes this allows it to maximize value for all its stakeholders. To learn more about PTC, please visit us at www.ptcbio.com and follow us on Facebook, Instagram, LinkedIn and X.
Forward-Looking Statement:
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements contained in this release, other than statements of historic fact, are forward-looking statements, including statements regarding: the future expectations, plans and prospects for PTC, expectations with respect to Kebilidi, including the timing of commercial availability; PTC's expectations with respect to the licensing, regulatory submissions and commercialization of its other products and product candidates; PTC's expectations with regards to its plans for the Rare Disease Priority Review Voucher; PTC's strategy, future operations, future financial position, future revenues, projected costs; and the objectives of management. Other forward-looking statements may be identified by the words, "guidance", "plan," "anticipate," "believe," "estimate," "expect," "intend," "may," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions.
PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward-looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the outcome of pricing, coverage and reimbursement negotiations with third party payors for PTC's products or product candidates that PTC commercializes or may commercialize in the future; expectations with respect to Kebilidi and Upstaza, including potential commercialization, any regulatory submissions and potential approvals, manufacturing capabilities and the potential achievement of development, regulatory and sales milestones and contingent payments that PTC may be obligated to make; significant business effects, including the effects of industry, market, economic, political or regulatory conditions; changes in tax and other laws, regulations, rates and policies; the eligible patient base and commercial potential of PTC's products and product candidates; PTC's scientific approach and general development progress; and the factors discussed in the "Risk Factors" section of PTC's most recent Annual Report on Form 10-K, as well as any updates to these risk factors filed from time to time in PTC's other filings with the SEC. You are urged to carefully consider all such factors.
As with any pharmaceutical under development, there are significant risks in the development, regulatory approval, and commercialization of new products. There are no guarantees that any product will receive or maintain regulatory approval in any territory, or prove to be commercially successful, including Kebilidi and Upstaza.
The forward-looking statements contained herein represent PTC's views only as of the date of this press release and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, estimates or projections, or other circumstances occurring after the date of this press release except as required by law.
References:
1. Wassenberg T, et al. Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency. Orphanet J Rare Dis. 2017;12(1):12.
2. Hwu WL et al. Natural History of Aromatic L -Amino Acid Decarboxylase Deficiency in Taiwan. JIMD Rep. 2018; 40:1-6.
3. Himmelreich N et al. Aromatic amino acid decarboxylase deficiency: Molecular and metabolic basis and therapeutic outlook. Mol Genet Metab. 2019;127:12–22
4. Chien YH, et al. Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1 /2 trial. Lancet Child Adolesc Health. 2017;1(4):265-273.
5. Pearson TS et al. AADC deficiency from infancy to adulthood: Symptoms and developmental outcome in an international cohort of 63 patients. J Inherit Metab Dis 2020;43:1121–30.
6. Chien YH, et al. 3-O-methyldopa levels in newborns: Result of newborn screening for aromatic L -amino-acid decarboxylase deficiency. Mol Genet Metab. August 2016;118(4):259-263.
7. Leuzzi, et al. Report of two never treated adult sisters with aromatic L-amino Acid decarboxylase deficiency: a portrait of the natural history of the disease or an expanding phenotype? JIMD Rep. 2015; 15: 39–45.
SOURCE PTC Therapeutics, Inc.
Posted: November 2024